Relative Atomic Mass of Magnesium
The M r of a hydrated salt eg magnesium sulfate by heating to constant mass. The ETA 6498 is used which began its life as a pocket watch movement for the manufacturer Unitas.

Magnesium Description Properties Compounds Britannica
The gives the number of moles of each.

. It became particularly famous in the early 1990s when Panerai equipped almost all models in the new line with it or with its direct relative 6497. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
All magnesium atoms in the nucleus have 12 protons. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. These values tell you that a magnesium atom has twice the mass of a carbon atom and 24 times more mass than a hydrogen atom.
The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. The term relative formula mass will be used for ionic compounds. From the source of.
AT a and k. Boost energy and aid restful sleep. They should divide mass by the atomic mass for each element.
Is time relative. Einsteins theory of. To measure the number of atoms in a sample you will figure out how many moles the sample element contains.
These values tell you that a magnesium atom has twice the mass of a carbon atom and 24 times more mass than a hydrogen atom. Students could be asked to find the percentage conversion of a Group 2 carbonate to its. The atomic number or nuclear charge number symbol Z of a chemical element is the charge number of an atomic nucleusFor ordinary nuclei this is equal to the proton number n p or the number of protons found in the nucleus for every atom of that element.
Magnesium is 24 and oxygen is 16. Best magnesium supplement 2022. The atomic number of uranium is 92 meaning that it has 92 protons and occupies place number 92 in the periodic table.
Relative atomic mass and relative molecular mass in terms of 12 C. Relative atomic mass The mass of an atom relative to that of carbon-12. The relative atomic mass of an element is a weighted average of.
A r of Mg 24 A r of N 14 A r of O 16 Reveal answer. The atomic weight is denoted by the symbol A r. Rajveer singh November 5 2019 at 321 pm.
In 1935 and 1936 Dempster Kenneth T. The atomic weight is also known as the relative atomic mass. They will also require the relative atomic masses.
The difference in mass can be calculated by the Einstein equation E mc 2 where E is the nuclear binding energy c is the speed of light and m is the difference in mass. This missing mass is known as the mass defect. If two peaks due to mass m and m Δm can just be separated the resolving power is mΔmThe early machines had resolving powers of only a few hundred.
The standard atomic weight of a chemical element symbol A r E for element E is the weighted arithmetic mean of the relative isotopic masses of all isotopes of that element weighted by each isotopes abundance on EarthFor example isotope 63 Cu A r 62929 constitutes 69 of the copper on Earth the rest being 65 Cu A r 64927 so Because relative. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. The relative atomic mass of an element.
All group 2. The most common isotope is U. Atomic mass is an absolute mass relative isotopic mass is a number without proportions and without units.
Its monatomic form H is the most abundant chemical substance in the Universe constituting. This is approximately the sum of the number of protons and neutrons in the nucleus. Electron configuration Ar 3d 6 4s 2.
Students should be able to. This became the famous Epsoms salt magnesium sulfate MgSO 4 and became a treatment for. 25 magnesium 24 potassium 20 and titanium 061.
The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. For a complete list see abundance of elements in Earths crust. The resolving power or resolution of a mass spectroscope is a measure of its ability to separate adjacent masses that are displayed as peaks on the detector.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Atomic Mass Of Calcium. Its equal to Avogadros number 602 X 1023 of atoms.
The only difference between them is that the magnesium-24 atom in the nucleus has 12 neutrons the magnesium-25 atom has 13 neutrons and the magnesium-26 atom has 14 neutrons. The relative atomic mass scale is used to compare the masses of different atoms. By using this chemists work out the chemical formula.
The average 70 kg 150 lb adult human body contains approximately 7 10 27 atoms and contains at least detectable traces of 60 chemical elements. Get 247 customer support help when you place a homework help service order with us. Atomic mass and Relative atomic mass Isotopes and radioactive decay isotopes half-life.
The movement is still being built today but the so-called hunter caliber was developed in the 1950s. Unlike the atomic mass the atomic weight does not have any unit. Calculate the relative formula mass M r of magnesium nitrate MgNO 3 2.
Like matter with negative mass or infinitely long cylinders. Its monatomic form H is the most abundant chemical substance in the Universe constituting. About 29 of these elements are thought to play an active positive role in life and health in humans.
The relative amounts of each element vary by individual mainly due to differences in the proportion of fat muscle and bone. The atomic number can be used to uniquely identify ordinary chemical elementsIn an ordinary uncharged atom the. The atomic mass is carried by the atomic nucleus which occupies only about 10 -12 of the total volume of the atom or less but it contains all the positive charge and at least 9995 of the total.
Magnesium is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column group 2 or alkaline earth metals of the periodic table. It occurs naturally in six isotopes U-233 to U-238 and therefore contains between 141 and 146 neutrons. The Earth retains oxygen as the second-largest component of its mass and largest atomic.
It is the weighted average atomic mass calculated using the relative abundance of isotopes of an element. The graph at right illustrates the relative atomic-abundance of the chemical elements in. Where more than one isotope exists the value given is the abundance weighted average.
Other elements occur at less than 015. 174 convert the given mass of a substance to the amount of the substance in moles and vice versa by using the relative atomic or formula mass. A mole is the choice of unit chemists.
The relative atomic mass of an element is a weighted average of. It is a dimensionless quantity. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply.
The mass of an atomic nucleus is less than the sum of the individual masses of the free constituent protons and neutrons.
Atomic Mass Of Elements Table Of First 30 Elements
What Is The Atomic Mass Of Magnesium Quora

What Is The Relative Atomic Mass And Relative Molecular Mass Of An Element A Plus Topper Https Www Aplusto Relative Atomic Mass Molecular Mass Molecular
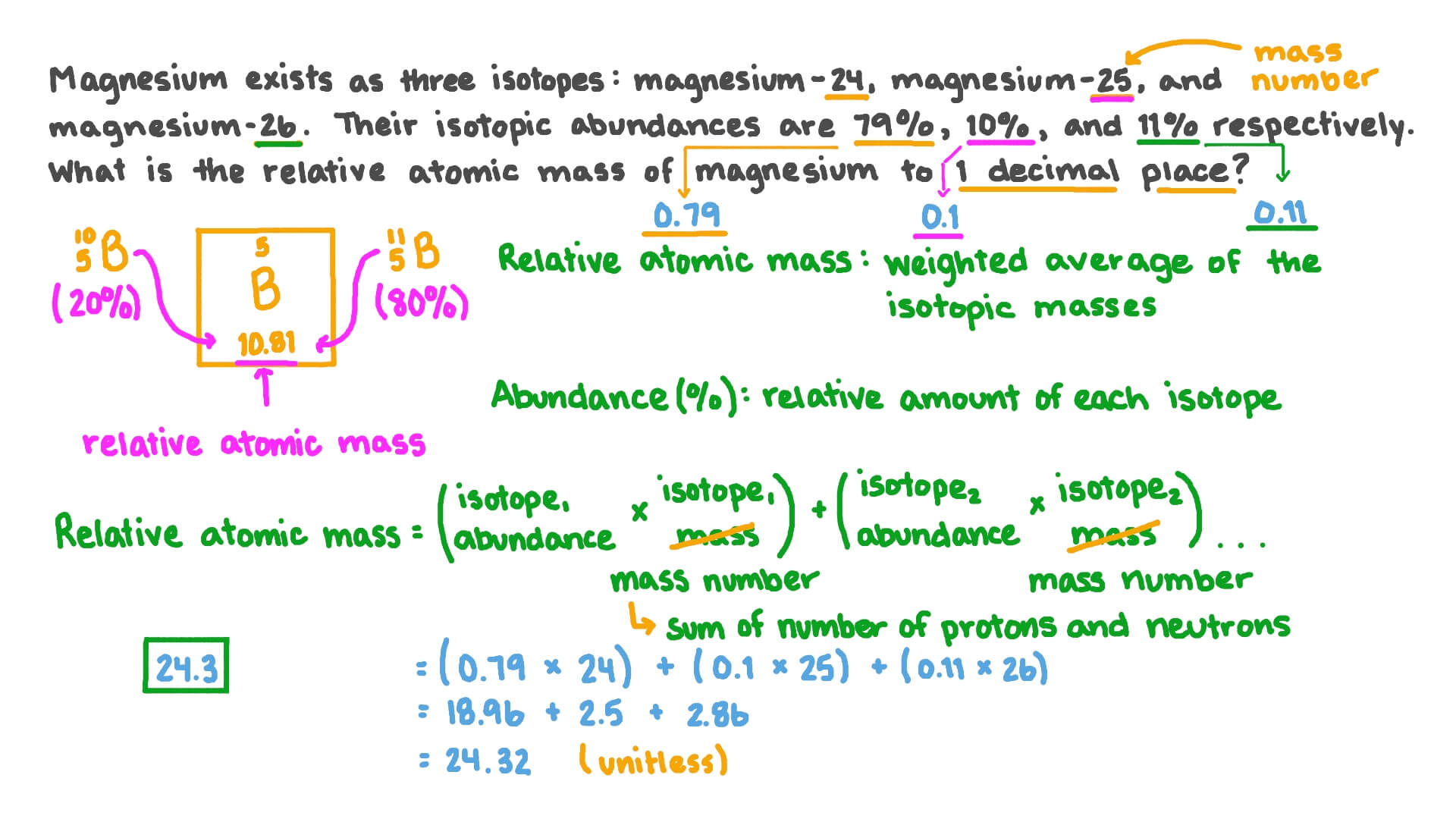
Question Video Calculating The Relative Atomic Mass Of Magnesium From Isotopic Abundances Nagwa
0 Response to "Relative Atomic Mass of Magnesium"
Post a Comment